The geology of mineral resources is intimately related to the entire geologic cycle, and nearly all aspects and processes of the cycle are involved to a lesser or greater extent in producing local concentrations of useful materials.
Local Concentrations of Metals
The term ore is sometimes used for those useful metallic minerals that can be mined at a profit, and locations where ore is found have anomalously high concentrations of these minerals. The concentration of metal necessary for a particular mineral to be classified as an ore varies with technology, economics, and politics. Before smelting (extraction of metal by heating) was invented, the only metal ores were those in which the metals appeared in their pure form; gold, for example, was originally obtained as a pure, or native, metal. Now gold mines extend deep beneath the surface, and the recovery process involves reducing tons of rock to ounces of gold. Although the rock contains only a minute amount of gold, we consider it a gold ore because we can extract the gold profitably. The concentration factor of a metal is the ratio of its necessary concentration for profitable mining (that is, of its concentration in ore) to its average concentration in the earth's crust. Aluminum has an average concentration of about 8 percent in the Earth's crust and needs to be found at concentrations of about 35 percent to be mined economically, giving it a concentration factor of about 4. Mercury, on the other hand, has an average concentration of only a tiny fraction of 1 percent and must have a concentration factor of about 10,000 to be mined economically. Nevertheless, mercury ores are common in certain regions, where they and other metallic ores are deposited. The percentage of a metal in ore (and thus the concentration factor) is subject to change as the demand for the metal changes.
Igneous Processes
Most ore deposits, caused by igneous processes, result from an enrichment process that concentrates an economically desirable ore of metals, such as copper, nickel, or gold. In some cases, however, an entire igneous rock mass contains disseminated crystals that can be recovered economically. Perhaps the best-known example is the occurrence of diamond crystals, found in a coarse-grained igneous rock called kimberlite, which characteristically occurs as a pipe shaped body of rock that decreases in diameter with depth. Almost the entire kimberlite pipe is the ore deposit, and the diamond crystals are disseminated throughout the rock. Diamonds, which are composed of carbon, form at very high temperatures and pressures, perhaps at depths as great as 150 km well below the crust of the earth and into the mantle. Some kimberlite pipes in South Africa are believed to be as old as 2 billion years. Near the surface, diamonds are not stable over geologic time and will eventually change to graphite (the mineral in lead pencils). The transformation will not happen at surface temperature and pressure, and, as a result, diamonds are metastable, remaining beautiful and mysterious for periods of time of interest to humans. The fact that the kimberlite pipes are so old suggests that they must be intruded (moved upward) from deep diamond forming depth to near the surface relatively quickly. If this were not the case, the diamonds would have been transformed to graphite.
Crystal Settling
More concentrated ore deposits can result from igneous processes called crystal settling that segregate crystals formed earlier from those formed later. For example, as magma cools, heavy minerals that crystallize early may slowly sink or settle toward the lower part of the magma chamber, where they form concentrated layers. Deposits of chromite (ore of chromium) have formed by this process.
Late Magmatic Processes and Hydrothermal Replacement
Late magmatic processes occur after most of the magma has crystallized, and rare and heavy metalliferous materials in water- and gas-rich solutions remain. This late-stage metallic solution may be squeezed into fractures or settle into interstices (empty spaces) between earlier-formed crystals. Other late-stage solutions form coarse-grained igneous rock known as pegmatite, which is rich in feldspar, mica, and quartz, as well as certain rare minerals. Pegmatites have been extensively mined for feldspar, mica, spodumene (lithium mineral), and clay that forms from weathered feldspar. Hydrothermal (hot-water) mineral deposits are a common type of ore deposit. They originate from late-stage magmatic processes and give rise to a variety of mineralization, including gold, silver, copper, mercury, lead, zinc, and other metals, as well as many non-metallic minerals. The hydrothermal solutions that form ore deposits are mineralizing fluids that migrate through a host rock, crystallizing as veins or small dikes. The mineral material is either produced directly from the igneous parent rock or altered by metamorphic processes, as magmatic solutions intrude into the surrounding rock (alteration by metamorphic processes, called contact metamorphism, is discussed under Metamorphic Processes). Many hydrothermal deposits cannot be traced to a parent igneous rock mass, however, and their origin remains unknown. It is speculated that circulating groundwater, heated and enriched with minerals after contact with deeply buried magma, might be responsible for some of these deposits.
Two types of hydrothermal deposits can be recognized: cavity-filling and replacement. Cavity-filling deposits are formed when hydrothermal solutions migrate along openings in rocks (such as fracture systems, pore spaces, or bedding planes) and precipitate (crystallize) ore minerals. Replacement deposits form as hydrothermal solutions react with the host rock, forming a zone in which ore minerals precipitate from the mineralizing fluids and replace part of the host rock. Although replacement deposits are believed to dominate at higher temperatures and pressures than cavity-filling deposits, both may be found in close association as one grades into the other; that is, the filling of an open fracture by precipitation from hydrothermal solutions may occur simultaneously with replacement of the rock that lines the fracture.
Hydrothermal replacement processes are significant because, excluding some iron and non-metallic deposits, they have produced some of the worlds largest and most important mineral deposits. Some of these deposits result from a massive, nearly complete replacement of host rock with ore minerals that terminate abruptly; others form thin replacement zones along fissures; and still others form disseminated replacement deposits that may involve huge amounts of relatively low-grade ore.
The actual sequence of geologic events leading to the development of a hydrothermal ore deposit is usually complex. Consider, for example, the tremendous, disseminated copper deposits of northern Chile. The actual mineralization is thought to be related to igneous activity, faulting, and folding that occurred 60 million to 70 million years ago. The ore deposit is an elongated, tabular mass along a highly sheared (fractured) zone separating two types of granitic rock.
The concentration of copper results from a number of factors:
- A source igneous rock supplied the copper.
- The fissure zone tapped the copper supply and facilitated movement of the mineralizing fluids.
- The host rock was altered and fractured, preparing it for deposition and replacement processes that produced the ore.
- The copper was leached and redeposited again by meteoric water, which further concentrated the ore.
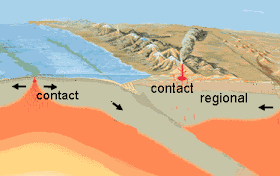
Metamorphic Processes
Ore deposits are often found along the contact between igneous rocks and the surrounding rocks they intrude. This area is characterized by contact metamorphism, caused by the heat, pressure, and chemically active fluids of the cooling magma interacting with the surrounding rock, called country, or host rock. The width of the contact metamorphic zone varies with the type of country rock. The zone is usually thickest in limestone because limestone is more reactive: The release of carbon dioxide increases the mobility of reactants. The zone is generally thinnest for shale because the fine-grained texture retards the movement of hot, chemically active solutions, and the zone is intermediate for sandstone. As we have already mentioned, some of the mineral deposits that form in contact areas originate from the magmatic fluids and some from reactions of these fluids with the country rock.
Regional Metamorphism
Metamorphism can also result from regional increase of temperature and pressure associated with deep burial of rocks or tectonic activity. This regional metamorphism can change the mineralogy and texture of the pre-existing rocks, producing ore deposits of asbestos, talc, graphite, and other valuable non-metallic deposits. Metamorphism has been suggested as a possible origin of some hydrothermal fluids. It is a particularly likely cause in high-temperature, high-pressure zones, where fluids might be produced and forced out into the surrounding rocks to form replacement or cavity-filling deposits. For example, the native copper found along the top of ancient basalt flows in the Michigan copper district was apparently produced by metamorphism and alteration of the basalt, which released the copper and other materials that produced the deposits.
Our discussion of igneous and metamorphic processes has focused primarily on ore deposits. However, igneous and metamorphic processes are also responsible for producing a good deal of stone used in the construction industry. Granite, basalt, marble (metamorphosed limestone), slate (metamorphosed shale), and quartzite (metamorphosed sandstone), along with other rocks, are quarried to produce crushed rock and dimension stone in the United States. Stone is used in many aspects of construction work; but many people are surprised to learn that, in total value, with the exception of iron and steel, the stone industry is one of the largest non-fuel mineral industries in the United States.
Sedimentary Processes
Sedimentary processes are often significant in concentrating economically valuable materials in sufficient amounts for extraction. As sediments are transported, wind and running water help segregate the sediment by size, shape, and density. Thus, the best sand or sand and gravel deposits for construction purposes are those in which the finer materials have been removed by water or wind. Sand dunes, beach deposits, and deposits in stream channels are good examples.
Sand and Gravel
The U.S. sand and gravel industry amounts to about $8.5 billion per year, and, by volume mined (about 1300 million tons in 2006), it is one of the largest non-fuel mineral industries in the United States. Currently, most sand and gravel are obtained from river channels and water-worked glacial deposits. The United States now produces more sand and gravel than it needs, but demand is increasing. Environmental restrictions on extraction are causing sand and gravel operations to move away from areas with high population density, and shortages of sand and gravel are expected to increase as zoning and land development restrict locations where they may be extracted. Extraction from river channels and active floodplains can cause degradation to the river environment, and objections to river extraction operations are becoming more common.
Placer Deposits
Stream processes transport and sort all types of materials according to size and density. Therefore, if the bedrock in a river basin contains heavy metals such as gold, streams draining the basin may concentrate heavy metals to form placer deposits (ore formed by deposit of sediments) in areas where there is reduced turbulence or velocity of flow, such as between particles on riffles, in open crevices
or fractures at the bottoms of pools, or at the inside curves of bends. Placer mining of gold known as a poor man method because a miner needed only a shovel, a pan, and a strong back to work the stream side claim helped to stimulate settlement of California, Alaska, and other areas of the United States. Furthermore, the gold in California attracted miners who acquired the expertise necessary to locate and develop other resources in the western conterminous United States and Alaska. Placer deposits of gold and diamonds have also been concentrated by coastal processes, primarily wave action. Beach sands and near-shore deposits are mined in Africa and other places.
Evaporite Deposits
Rivers and streams that empty into the oceans and lakes carry tremendous quantities of dissolved material derived from the weathering of rocks. From time to time, geologically speaking, a shallow marine basin may be isolated by tectonic activity (uplift) that restricts circulation and facilitates evaporation. In other cases, climatic variations during the ice ages produced large inland lakes with no outlets, which eventually dried up. In either case, as evaporation progresses, the dissolved materials precipitate, forming a wide variety of compounds, minerals, and rocks called evaporite deposits that have important commercial value.
Most evaporite deposits can be grouped into one of three types:
Marine evaporites (solids) potassium and sodium salts, calcium carbonate, gypsum, and anhydrite; non-marine evaporites (solids) sodium and calcium carbonate, sulphate, borate, nitrate, and limited iodine and strontium compounds; and brines (liquids derived from wells, thermal springs, inland salt lakes, and seawaters) bromine, iodine, calcium chloride, and magnesium. Heavy metals (such as copper, lead, and zinc) associated with brines and sediments in the Red Sea, Salton Sea, and other areas are important resources that may be exploited in the future. Extensive marine evaporite deposits exist in the United States. The major deposits are halite (common salt, NaCl), gypsum anhydrite , and inter-bedded limestone Limestone, gypsum, and anhydrite are present in nearly all marine evaporite basins, and halite and potassium minerals are found in a few. Evaporite materials are widely used in industry and agriculture. Marine evaporites can form stratified deposits that may extend for hundreds of kilometres, with a thickness of several thousand meters. The evaporites represent the product of evaporation of seawater in isolated shallow basins with restricted circulation. Within many marine evaporite basins, the different deposits are arranged in broad zones that reflect changes in salinity and other factors controlling the precipitation of evaporites; that is, different materials may be precipitated at the same time in different parts of the evaporite basin. Halite, for example, is precipitated in areas where the brine is more saline, and gypsum where it is less saline. Economic deposits of potassium evaporite minerals are relatively rare but may form from highly concentrated brines. Non-marine evaporite deposits form by evaporation of lakes in a closed basin. Tectonic activity, such as faulting, can produce an isolated basin with internal drainage and no outlet. However, to maintain a favourable environment for evaporite mineral precipitation, the tectonic activity must continue to uplift barriers across possible outlets or lower the basin floor faster than sediment can raise it. Even under these conditions, economic deposits of evaporites will not form unless sufficient dissolved salts have washed into the basin by surface run off from surrounding highlands. Finally, even if all favourable environmental criteria are present, including an isolated basin with sufficient run off and dissolved salts, valuable non-marine evaporates, such as sodium carbonate or borate, will not form unless the geology of the highlands surrounding the basin is also favourable and yields run off with sufficient quantities of the desired material in solution. Some evaporite beds are compressed by overlying rocks and mobilized, then pierce or intrude the overlying rocks. Intrusions of salt, called salt domes, are quite common in the Gulf Coast of the United States and are also found in north western Germany, Iran, and other areas. Salt domes in the Gulf Coast are economically important because:
- They are a good source for nearly pure salt. Some have extensive deposits of elemental sulphur.
- Some have oil reserves on their flanks.
Salt domes are also environmentally important as possible permanent disposal sites for radioactive waste, although, because salt domes tend to be mobile, their suitability as disposal sites for hazardous wastes must be seriously questioned. Evaporites from brine resources of the United States are substantial, assuring that no shortage is likely for a considerable period of time. But many evaporites will continue to have a place value because transportation of these mineral commodities increases their price, so continued discoveries of high-grade deposits closer to where they will be consumed remains an important goal.
Biological Processes
Organisms are able to form many kinds of minerals, such as the various calcium and magnesium carbonate minerals in shells and calcium phosphate in bones. Some of these minerals cannot be formed inorganically in the biosphere. Thirty-one different biologically produced minerals have been identified. Minerals of biological origin contribute significantly to sedimentary deposits.
An interesting example of mineral deposits produced by biological processes are phosphates associated with sedimentary marine deposition. Phosphorus-rich sedimentary rocks are fairly common in some of the western states, as well as in Tennessee, North Carolina, and Florida. The common phosphorus-bearing mineral in these rocks is apatite, a calcium phosphate associated with bones and teeth. Fish and other marine organisms extract the phosphate from seawater to form apatite, and the mineral deposit results from sedimentary accumulations of the phosphate-rich fish bones and teeth. The richest phosphate mine in the world, known as Bone Valley, is located about 40 km east of Tampa, Florida. The deposit is marine sedimentary rocks composed in part of fossils of marine animals that lived 10 million to 15 million years ago, when Bone Valley was the bottom of a shallow sea. That deposit has supplied as much as one-third of the worlds phosphate production. Another important source of phosphorus is guano (bird faeces), which accumulates where there are large colonies of nesting sea birds and a climate arid enough for the guano to dry to a rock like mass. Thus, the formation of one of the major sources of phosphorus depends upon unique biological and geographical conditions.
Weathering Processes
Weathering is responsible for concentrating some materials to the point that they can be extracted at a profit. Weathering processes can produce residual ore deposits in the weathered material and provide secondary enrichment of low-grade ore.
Residual Ore Deposits
Intensive weathering of rocks and soils can produce residual deposits of the less soluble materials, which may have economic value. For example, intensive weathering of some rocks forms a type of soil known as laterite (a residual soil derived from aluminium- and iron-rich igneous rocks). The weathering processes concentrate relatively insoluble hydrated oxides of aluminium and iron, while more soluble elements, such as silica, calcium, and sodium, are selectively removed by soil and biological processes. If sufficiently concentrated, residual aluminium oxide forms an aluminium ore known as bauxite. Important nickel and cobalt deposits are also found in laterite soils, developed from ferromagnesian-rich igneous rocks. Insoluble ore deposits, such as native gold, are generally residual, meaning that, unless they are removed by erosion, they accumulate in weathered rock and soil. Accumulation of the insoluble ore minerals is favoured where the parent rock is a relatively soluble material, such as limestone. Care must be taken in evaluating a residual weathered rock or soil deposit because the near-surface concentration may be a much higher grade than ore in the parent, un-weathered rocks.
Secondary Enrichment
Weathering is also involved in secondary enrichment processes to produce sulphide ore deposits from low-grade primary ore. Near the surface, primary ore containing such minerals as iron, copper, and silver sulphides is in contact with slightly acid soil water in an oxygen-rich environment. As the sulphides are oxidized, they are dissolved, forming solutions rich in sulphuric acid and in silver and copper sulphate; these solutions migrate downward, producing a leached zone devoid of ore minerals. Below the leached zone, oxidation continues, as the sulphate solutions continue to move toward the groundwater table. Below the water table, if oxygen is no longer available, the solutions are deposited as sulphides, increasing the metal content of the primary ore as much as tenfold. In this way, low-grade primary ore is rendered more valuable, and high-grade primary ore is made even more attractive.
The presence of a residual iron oxide cap at the surface indicates the possibility of an enriched ore below, but it is not always conclusive. Of particular importance to the formation of a zone of secondary enrichment is the presence in the primary ore of iron sulphide (for example, pyrite). Without it, secondary enrichment seldom takes place, because iron sulphide in the presence of oxygen and water forms sulphuric acid, which is a necessary solvent. Another factor favouring development of a secondary-enrichment ore deposit is the primary ore being sufficiently permeable to allow water and solutions to migrate freely downward. Given a primary ore that meets these criteria, the reddish iron oxide cap probably does indicate that secondary enrichment has taken place.
Several disseminated copper deposits have become economically successful because of secondary enrichment, which concentrates dispersed metals. For example, secondary enrichment of a disseminated copper deposit at Miami, Arizona, increased the grade of the ore from less than 1 percent copper in the primary ore to as much as 5 percent in some localized zones of enrichment.
Other Minerals from the Sea
Mineral resources in seawater or on the bottom of the ocean are vast and, in some cases, such as magnesium, nearly unlimited. In the United States, magnesium was first extracted from seawater in 1940. By 1972, one company in Texas produced 80 percent of our domestic magnesium, using seawater as its raw material source. In 1992, three companies in Texas, Utah, and Washington extracted magnesium, respectively, from seawater, lake brines, and dolomite (mineral composed of calcium and magnesium carbonate). The deep-ocean floor may eventually be the site of a next mineral rush. Identified deposits include massive sulphide deposits associated with hydrothermal vents, manganese oxide nodules, and cobalt-enriched manganese crusts.
Sulfide Deposits
Massive sulphide deposits containing zinc, copper, iron, and trace amounts of silver are produced at divergent plate boundaries (oceanic ridges) by the forces of plate tectonics. Pressure created by several thousand meters of water at ridges forces cold seawater deep into numerous rock fractures, where it is heated by up-welling magma to temperatures as high as The pressure of the heated water produces vents known as black smokers, from which the hot, dark-coloured, mineral-rich water emerges as hot springs. Circulating seawater leaches the surrounding rocks, removing metals that are deposited when the mineral-rich water is ejected into the cold sea. Sulphide minerals precipitate near the vents, forming massive tower like formations, rich in metals. The hot vents are of particular biologic significance because they support a unique assemblage of animals, including giant clams, tube worms, and white crabs. Ecosystems including these animals base their existence on sulphide compounds extruded from black smokers, existing through a process called chemosynthesis, as opposed to photosynthesis, which supports all other known ecosystems on earth. The extent of sulphide mineral deposits along oceanic ridges is poorly known, and, although leases to some possible deposits are being considered, it seems unlikely that such deposits will be extracted at a profit in the near future. Certainly, potential environmental degradation, such as decreased water quality and sediment pollution, will have to be carefully evaluated prior to any mining activity. Study of the formation of massive sulphide deposits at oceanic ridges is helping geologists understand some of the mineral deposits on land. For example, massive sulphide deposits being mined in Cyprus are believed to have formed at an oceanic ridge and to have been later uplifted to the surface.
Manganese Oxide Nodules
Manganese oxide nodules cover vast areas of the deep-ocean floor. They contain manganese (24 percent) and iron (14 percent), with secondary copper (1 percent), nickel (1 percent), and cobalt (0.25 percent). Nodules are found in the Atlantic Ocean off Florida, but the richest and most extensive accumulations occur in large areas of the north eastern, central, and southern Pacific, where they cover 20 to 50 percent of the ocean floor.
Manganese oxide nodules are usually discrete, but are welded together locally to form a continuous pavement. Although they are occasionally found buried in sediment, nodules are usually surficial deposits on the seabed. Their size varies from a few millimetres to a few tens of centimetres in diameter (many are marble to baseball sized). Composed primarily of concentric layers of manganese and iron oxides, mixed with a variety of other materials, each nodule formed around a nucleus of a broken nodule, a fragment of volcanic rock, or, sometimes, a fossil. The estimated rate of nodular growth is 1 to 4 mm per million years. The nodules are most abundant in those parts of the ocean where sediment accumulation is at a minimum, generally at depths of 5 to 7 km.
The origin of the nodules is not well understood; presumably, they might form in several ways. The most probable theory is that they form from material weathered from the continents and transported by rivers to the oceans, where ocean currents carry the material to the deposition site in the deep-ocean basins. The minerals from which the nodules form may also derive from submarine volcanism, or may be released during physical and biochemical processes and reactions that occur near the water sediment interface during and after deposition of the sediments. Mining of manganese oxide nodules involves lifting the nodules off the bottom and up to the mining ship; this may be done by using suction or scraper equipment. Although mining of the nodules appears to be technologically feasible, production would be expensive compared to mining manganese on land. In addition, there are uncertainties concerning ownership of the nodules, and nodule mining would cause significant damage to the sea-floor and local water quality, raising environmental concerns.
Cobalt-enriched Manganese Crusts
Oceanic crusts rich in cobalt and manganese are present in the mid- and south-west Pacific, on flanks of sea-mounts, volcanic ridges, and islands. Cobalt content varies with water depth; the maximum concentration of about 2.5 percent is found at water depths of 1 to 2.5 km. Thickness of the crust averages about 2 cm. The processes of formation are not well understood. Both the nature and the extent of the crusts, which also contain nickel, platinum, copper, and molybdenum, are being studied by U.S. Geological Survey scientists.