Many different materials may pollute surface water or groundwater. We will focus on oxygen-demanding waste, pathogenic organisms, nutrients, oil, hazardous chemicals, heavy metals, radioactive materials, and sediment.
Oxygen-Demanding Waste
Dead organic matter in streams decays; that is, it is consumed by bacteria, which require oxygen. If there is enough bacterial activity, the oxygen in the water can be reduced to levels so low that fish and other organisms die. A stream without oxygen is a dead stream, devoid of fish and many organisms we value. The amount of oxygen used for bacterial decomposition is the biochemical oxygen demand (BOD), a commonly used measure in water quality management. The BOD is measured as milligrams per litre of oxygen consumed over five days at 20° C. A high BOD indicates a high level of decaying organic matter in the water.
Dead organic matter in streams and rivers comes from natural sources (for example, dead leaves from a forest), as well as from agriculture and urban sewage. Approximately 33 percent of all BOD in streams results from agricultural activities, but urban areas, particularly those with sewer systems that combine sewage and storm-water run off, may add considerable BOD to streams during floods, when sewers entering treatment plants can be overloaded and overflow into streams, producing pollution events.
The threshold for water pollution is a dissolved oxygen content of less than 5 mg per litre (mg/l) of water. The diagram in Figure above illustrates the effect of BOD on dissolved oxygen content in a stream when raw sewage is introduced as a result of an accidental spill. Three zones are recognised. The pollution zone has a high BOD and a reduced dissolved oxygen content as initial decomposition of the waste begins. In the active decomposition zone, the dissolved oxygen content is at a minimum, owing to biochemical decomposition as the organic waste is transported downstream. In the recovery zone, the dissolved oxygen increases, and the BOD is reduced because most oxygen demanding organic waste from the input of sewage has decomposed and natural stream processes are replenishing the water with dissolved oxygen. All streams have some capability to degrade organic waste after it enters the stream. Problems result when the stream is overloaded with biochemical oxygen-demanding waste, overpowering the streams natural cleansing function.
Dead organic matter in streams and rivers comes from natural sources (for example, dead leaves from a forest), as well as from agriculture and urban sewage. Approximately 33 percent of all BOD in streams results from agricultural activities, but urban areas, particularly those with sewer systems that combine sewage and storm-water run off, may add considerable BOD to streams during floods, when sewers entering treatment plants can be overloaded and overflow into streams, producing pollution events.
 |
| Relationship between dissolved oxygen and biochemical oxygen demand (BOD) for a stream, following the input of sewage. |
Pathogenic Organisms
Pathogenic (disease-causing) micro-organisms are important biological pollutants. Among the major water borne human diseases are cholera, typhoid infections, hepatitis, and dysentery. Because it is often difficult to monitor the pathogens directly, we use the count of human faecal coliform bacteria as a common measure of biological pollution and a standard measure of microbial pollution. These common and, usually, harmless bacteria are normal constituents of human intestines and are found in all human waste.
However, not all forms of faecal coliform bacteria are harmless. Escherichia coli (also known as E. coli 0157), a strain of E. coli bacteria, has been responsible for many human illnesses and deaths. E. coli 0157 produces strong toxins in humans that may lead to bloody diarrhea, dehydration, kidney failure, and death. In 1993, outbreaks of disease, apparently caused by E. coli 0157, occurred as a result of peoples eating contaminated meat at a popular fast-food restaurant. In 1998, E. coli apparently contaminated the water in a Georgia water park and a Wyoming towns water supply, causing illness and one death.
One of the worst outbreaks of E. coli bacterial infection in Canadian history unfolded in May 2000 in Walkerton, Ontario. It is believed that the likely cause of the contamination in Walkerton was the result of E. coli bacteria in cow manure that washed into the public water supply during heavy rains and flooding that occurred on May 12, 2000. The local Public Utility Commission was aware as early as May 18 that water from wells serving the town was contaminated, but they did not report this contamination immediately to health authorities. As a result, people were not advised to boil water until it was too late to avoid the outbreak of disease. By May 26, 5 people had died, over 20 were in the intensive care unit of the local hospital, and approximately 700 were ill with severe symptoms, including cramps, vomiting, and diarrhoea. The old and very young are most vulnerable to the ravages of the disease, which can damage the kidneys, and two of the first victims were a 2-year-old baby and an 82-year-old woman. Government officials finally took over management of the water supply, and bottled water was distributed. Tragically, before the outbreak was over, at least 7 people had died and over 1000 had been infected.
Authorities launched an investigation, focusing on why there was such a long delay between identifying the potential problem and warning people. Had there not been such a long delay, illnesses might have been avoided. We must remain vigilant in testing our waters and immediately report problems to public health authorities if any problems arise.
In the fall of 2006, E. coli 0157 was traced to farms in northern California. Contaminated spinach was shipped to 23 states. About 150 people became sick and one person died. In 2009, peanut butter was responsible for several hundred E. coli illnesses, with several deaths across the United States.
In the past, epidemics of water borne diseases have killed thousands of people in U.S. cities. Such epidemics have been largely eliminated by separating sewage water and drinking water and treating drinking water before consumption. Unfortunately, this is not the case worldwide, and, every year, several billion people, particularly in poor countries, are exposed to water borne diseases. For example, an epidemic of cholera occurred in South America in the 1990s. Although developing nations are more vulnerable, the risk of water borne diseases is a potential threat in all countries.
The threat of an outbreak of a water borne disease is exacerbated by disasters such as earthquakes, floods, and hurricanes; these events can damage sewer lines or cause them to overflow, resulting in contamination of water supplies. For example, after the 1994 North ridge earthquake, people in the San Fernando Valley of the Los Angeles Basin were advised to purify municipal water by boiling because of the threat of bacterial contamination.
However, not all forms of faecal coliform bacteria are harmless. Escherichia coli (also known as E. coli 0157), a strain of E. coli bacteria, has been responsible for many human illnesses and deaths. E. coli 0157 produces strong toxins in humans that may lead to bloody diarrhea, dehydration, kidney failure, and death. In 1993, outbreaks of disease, apparently caused by E. coli 0157, occurred as a result of peoples eating contaminated meat at a popular fast-food restaurant. In 1998, E. coli apparently contaminated the water in a Georgia water park and a Wyoming towns water supply, causing illness and one death.
One of the worst outbreaks of E. coli bacterial infection in Canadian history unfolded in May 2000 in Walkerton, Ontario. It is believed that the likely cause of the contamination in Walkerton was the result of E. coli bacteria in cow manure that washed into the public water supply during heavy rains and flooding that occurred on May 12, 2000. The local Public Utility Commission was aware as early as May 18 that water from wells serving the town was contaminated, but they did not report this contamination immediately to health authorities. As a result, people were not advised to boil water until it was too late to avoid the outbreak of disease. By May 26, 5 people had died, over 20 were in the intensive care unit of the local hospital, and approximately 700 were ill with severe symptoms, including cramps, vomiting, and diarrhoea. The old and very young are most vulnerable to the ravages of the disease, which can damage the kidneys, and two of the first victims were a 2-year-old baby and an 82-year-old woman. Government officials finally took over management of the water supply, and bottled water was distributed. Tragically, before the outbreak was over, at least 7 people had died and over 1000 had been infected.
Authorities launched an investigation, focusing on why there was such a long delay between identifying the potential problem and warning people. Had there not been such a long delay, illnesses might have been avoided. We must remain vigilant in testing our waters and immediately report problems to public health authorities if any problems arise.
In the fall of 2006, E. coli 0157 was traced to farms in northern California. Contaminated spinach was shipped to 23 states. About 150 people became sick and one person died. In 2009, peanut butter was responsible for several hundred E. coli illnesses, with several deaths across the United States.
In the past, epidemics of water borne diseases have killed thousands of people in U.S. cities. Such epidemics have been largely eliminated by separating sewage water and drinking water and treating drinking water before consumption. Unfortunately, this is not the case worldwide, and, every year, several billion people, particularly in poor countries, are exposed to water borne diseases. For example, an epidemic of cholera occurred in South America in the 1990s. Although developing nations are more vulnerable, the risk of water borne diseases is a potential threat in all countries.
The threat of an outbreak of a water borne disease is exacerbated by disasters such as earthquakes, floods, and hurricanes; these events can damage sewer lines or cause them to overflow, resulting in contamination of water supplies. For example, after the 1994 North ridge earthquake, people in the San Fernando Valley of the Los Angeles Basin were advised to purify municipal water by boiling because of the threat of bacterial contamination.
Nutrients
 |
| Relationship between land use and average nitrogen and phosphorus concentration in streams (in milligrams per liter). |
Nutrients released by human activity may lead to water pollution. Two important nutrients that can cause problems are phosphorus and nitrogen, both of which are released from a variety of materials, including fertilizers, detergents, and the products of sewage-treatment plants. The concentration of phosphorus and nitrogen in streams is related to land use. Forested land has the lowest concentrations of phosphorus and nitrogen, while the highest concentrations are found in agricultural areas, such as fertilized farm fields and feed lots. Urban areas can also add phosphorus and nitrogen to local waters, particularly where waste water treatment plants discharge treated waters into rivers, lakes, or the ocean. These plants are effective in reducing organic pollutants and pathogens, but, without advanced treatment, nutrients pass through the system.
High human-caused concentrations of nitrogen and phosphorus in water often result in the process known as cultural eutrophication. Eutrophication (from the Greek for well fed ), a natural process, is characterized by a rapid increase in the abundance of plant life, particularly algae. Blooms of algae form thick mats that sometimes nearly cover the surface of the water in freshwater ponds and lakes. The algae block sunlight to plants below, and the plants eventually die. In addition, the algae consume oxygen as they decompose, thereby lowering the oxygen content of the water, and fish and aquatic animals may die as well.
Algae blooms from blue-green algae may produce toxins as part of their life cycle. Lakes in Wisconsin, Minnesota, Oregon, and other areas with blooms of blue-green algae turn pea green, and toxins that are produced have been responsible for deaths of dogs and other animals that drink the water. People who live near the lake have reported nauseating smells from the water, along with rashes, headaches, and sore throats. People have not been killed by the toxins because they generally avoid contact with the water.
High human-caused concentrations of nitrogen and phosphorus in water often result in the process known as cultural eutrophication. Eutrophication (from the Greek for well fed ), a natural process, is characterized by a rapid increase in the abundance of plant life, particularly algae. Blooms of algae form thick mats that sometimes nearly cover the surface of the water in freshwater ponds and lakes. The algae block sunlight to plants below, and the plants eventually die. In addition, the algae consume oxygen as they decompose, thereby lowering the oxygen content of the water, and fish and aquatic animals may die as well.
Algae blooms from blue-green algae may produce toxins as part of their life cycle. Lakes in Wisconsin, Minnesota, Oregon, and other areas with blooms of blue-green algae turn pea green, and toxins that are produced have been responsible for deaths of dogs and other animals that drink the water. People who live near the lake have reported nauseating smells from the water, along with rashes, headaches, and sore throats. People have not been killed by the toxins because they generally avoid contact with the water.
 | |
| Dead zone in Gulf of Mexico Area in the Gulf of Mexico in July 2001 with bottom water with less than 2 mg/L dissolved oxygen. |
A serious and ongoing cultural eutrophication problem is occurring in the Gulf of Mexico, offshore of Louisiana. A so-called dead zone develops in the summer, over a large area about the size of New Jersey. Water in the zone has low concentrations of oxygen, killing shellfish and crabs, and blooms of algae occur. The cause of the cultural eutrophication is believed to be the Mississippi River. The Mississippi drains about 40 percent of the lower 48 states, and much of the land use in the drainage basin is agricultural. The nutrient believed to cause the problem is nitrogen, which is used in great amounts to fertilize fields. The problem will not be easy to solve, as long as agriculture continues to use tremendous amounts of fertilizer. Part of the solution will be modification of agricultural practices to use less nitrogen by using it more efficiently, so that less of the nutrient runs off the land into the river.
Oil
Oil discharged into surface water (rivers, lakes, and the ocean) has caused major pollution problems. The largest oil discharges have usually involved oil-tanker accidents at sea. For example, just after midnight on March 24, 1989, the oil tanker Exxon Valdez ran aground on Bligh Reef, 40 km (25 mi) south of Valdez, Alaska, in Prince William Sound. Crude oil poured out of the ruptured tanks of the vessel at a rate of approximately 20,000 barrels per hour. The Exxon Valdez was loaded with 1.2 million barrels of crude oil, and, of this, more than 250,000 barrels (11 million gallons) gushed from the hold of the 300-m (984-ft) tanker. The oil remaining in the Exxon Valdez was loaded into another tanker.
The oil spilled into what was considered one of the most pristine and ecologically rich marine environments of the world, and the accident is now known as the worst oil spill in the history of the United States. Short term impacts were very significant; commercial fisheries, sport fisheries, and tourism were disrupted. In addition, many sea birds and mammals were lost. Lessons learned from the Exxon Valdez spill have resulted in better management strategies for both the shipment of crude oil and emergency plans to minimize environmental degradation.
 |
| Mercury in the environment Input and changes of mercury in aquatic ecosystems. |
A large oil spill in 2006 was caused by the war in Lebanon, when a coastal power plant was bombed and over 100,000 barrels of fuel oil entered the Mediterranean Sea. Over half of Lebanon's tourist beaches were polluted, including a popular public beach visited by people from the capital city Beirut.
Toxic Substances
Many substances that enter surface water and groundwater are toxic to organisms. Three general categories of toxic substances synthetic organic chemicals, heavy metals, and radioactive waste will be discussed.
Synthetic Organic Chemicals
Organic compounds are compounds of carbon that are produced naturally by living organisms or synthetically by industrial processes. Up to 100,000 new chemicals are now being used or have been used in the past. It is difficult to generalize concerning the environmental and health effects of synthetic organic compounds because there are so many of them and they have so many uses and produce so many different effects.
Synthetic organic compounds have many uses in industrial processes, including pest control, pharmaceuticals, and food additives. Some of these compounds are called persistent organic pollutants, also known as POPs. Many of these chemicals were produced decades ago, before their harm to the environment was known, and a number have now been banned or restricted. Table above lists some of the common persistent organic pollutants and their uses. POPs have several general properties useful in defining them.First, they have a carbon-based structure and often contain reactive chlorine. Second, most are produced by human processes and, thus, are synthetic chemicals. Third, they persist in the environment, do not break down easily, are polluting and toxic, and tend to accumulate in living tissue. Fourth, they occur in a number of forms that allow them to be easily transported by water and wind, with sediment, for long distances.
A significant example of water polluter is the chemical MTBE (methyl tertbutyl ether). The Clean Air Act Amendments that were passed in 1990 required cities with air pollution problems to use what are known as oxygen additives in gasoline. MTBE is added to gasoline with the objective of increasing the oxygen level of the gasoline and decreasing emissions of carbon monoxide from gasoline-burning cars. It is used because MTBE is more economical than other additives, including alcohol. MTBE is very soluble in water and is a commonly detected volatile organic compound (VOC) in urban groundwater. It is hypothesized that the MTBE detected in shallow groundwater originates from three sources: urban storm-water runoff, leaking underground gasoline tanks, and leakage occurring at service stations when car tanks are being filled.
It is ironic that a gasoline additive intended to improve air quality contaminated the groundwater that was used as a source of drinking water for approximately 15 million people in California. In 1997, MTBE-polluted groundwater in Santa Monica, California, forced the city to stop pumping groundwater, eliminating approximately 50 percent of the total drinking water supply for the city. Concentrations of MTBE in Santa Monicas groundwater ranged from about 8 to 600 micrograms per litre. The Environmental Protection Agency has stated that concentrations of 20 to 40 of MTBE per litre of water are sufficient to cause objectionable taste and odour. MTBE in that concentration smells like turpentine or fresh paint and is nauseating to some people. Studies are under way concerning the toxicity of MTBE, and some researchers fear it is a carcinogenic chemical. As a result of the contamination, some states, such as California, have terminated the use of MTBE. Many other states followed and, by 2006, MTBE was all but phased out in the United States. However, MTBE remains a groundwater pollution problem that can contaminate surface water (see Putting Some Numbers on Water Pollution). Figure above illustrates some of the pathways of MTBE, as well as other volatile organic compounds in the hydrologic cycle of an urban area.
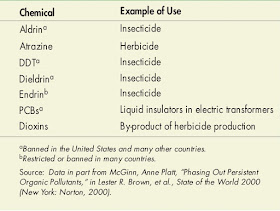 |
| Selected Persistent Organic Pollutants (POPs). |
A significant example of water polluter is the chemical MTBE (methyl tertbutyl ether). The Clean Air Act Amendments that were passed in 1990 required cities with air pollution problems to use what are known as oxygen additives in gasoline. MTBE is added to gasoline with the objective of increasing the oxygen level of the gasoline and decreasing emissions of carbon monoxide from gasoline-burning cars. It is used because MTBE is more economical than other additives, including alcohol. MTBE is very soluble in water and is a commonly detected volatile organic compound (VOC) in urban groundwater. It is hypothesized that the MTBE detected in shallow groundwater originates from three sources: urban storm-water runoff, leaking underground gasoline tanks, and leakage occurring at service stations when car tanks are being filled.
 | |
| Pathways for chemical pollutants within the hydrologic cycle of the environment. |
Heavy Metals
Heavy metals, such as lead, mercury, zinc, cadmium, and arsenic, are dangerous pollutants that are often deposited with natural sediment in the bottoms of stream channels. If these metals are deposited on floodplains, they may become incorporated into plants, including food crops, and animals. Once the metal has dissolved in water used for agricultural or domestic use, heavy-metal poisoning can result.
As an example, consider mercury contamination of aquatic ecosystems. It has been known for decades that mercury is a significant pollutant of aquatic ecosystems, including ponds, lakes, rivers, and the ocean.
Perhaps the best-known case history of mercury toxicity comes from Minamata, Japan. Minamata is a coastal town on the island of Kyushu and was the site of a serious illness that was first recognized in the middle of the twentieth century.
It was first called the disease of the dancing cats because the illness was first observed in cats that seemingly went mad and ran in circles, foaming at the mouth. It was also noticed that birds flew into buildings or fell to the ground. People were subsequently affected, most being families of fishermen. Some of the first symptoms were fatigue, irritability, numbness in arms and legs, and headaches, as well as difficulty in swallowing. Some of the more severe symptoms included blurred vision, loss of hearing, and loss of muscular coordination. Some people complained of a metallic taste in their mouths and suffered from diarrhoea. By the time the disease ran its course, over 40 people died and over 100 were severely disabled. The people affected by the disease lived in a relatively small area, and their diet mostly came from fish harvested from Minamata Bay.
The disease was eventually traced to a vinyl chloride factory on Minamata Bay that used mercury in its production processes. Inorganic mercury was released as waste into the bay, and it was believed that the mercury would not get into the food chain. However, the inorganic mercury was converted by bacterial activity in Minamata Bay to methyl mercury. Methyl mercury readily passes through cell membranes and is transported throughout the body by red blood cells. It can enter and damage brain cells. The harmful effects of methyl mercury depend on a number of factors that include the amount of exposure and intake, the duration of the exposure, and the species affected. The effects of the mercury are often delayed from several weeks to months in people from the time of ingestion. Furthermore, if the intake of mercury ceases, some of the symptoms may disappear, but others are difficult to reverse.
The disease of the dancing cats eventually became known as Minamata disease, and nearly 800 people were officially recognized as having the disease but as many as several thousand may have been involved. The mercury pollution in the bay ceased in 1968. As recently as the 1990s, some of the people afflicted by the disease were still being compensated for damages.
Arsenic is an example of a highly toxic natural metal that is found in soil, rock, and water. There are many industrial and commercial uses of arsenic compounds, including the processing of glass, pesticides, and wood preservatives. Arsenic may enter our water supplies through a number of processes, including natural rain, snow melt, or groundwater flow. It may also be released with industrial waste water and agricultural processes. Finally, it may be released through the production of pesticides, the burning of fossil fuels, and as a by-product of mining.
Arsenic has been known as a deadly poison since ancient times, and, more recently, it has been recognized that elevated levels of arsenic in drinking water may cause a variety of health problems that affect organs such as the bladder, lung, and kidney. It may also cause disease to the central nervous system. Finally, arsenic is known to be a carcinogen (capable of causing or promoting cancer).
The occurrence of arsenic in drinking water is now recognized as a global problem. It certainly is not found in all water supplies, but it is found in many around the world. For example, arsenic in groundwater in Bangladesh has affected many millions of some of the poorest people on Earth. Ongoing research has the objective of identifying those locations where arsenic pollution occurs and of developing appropriate technology or methods to avoid or reduce the hazard of exposure to arsenic.
As an example, consider mercury contamination of aquatic ecosystems. It has been known for decades that mercury is a significant pollutant of aquatic ecosystems, including ponds, lakes, rivers, and the ocean.
Perhaps the best-known case history of mercury toxicity comes from Minamata, Japan. Minamata is a coastal town on the island of Kyushu and was the site of a serious illness that was first recognized in the middle of the twentieth century.
It was first called the disease of the dancing cats because the illness was first observed in cats that seemingly went mad and ran in circles, foaming at the mouth. It was also noticed that birds flew into buildings or fell to the ground. People were subsequently affected, most being families of fishermen. Some of the first symptoms were fatigue, irritability, numbness in arms and legs, and headaches, as well as difficulty in swallowing. Some of the more severe symptoms included blurred vision, loss of hearing, and loss of muscular coordination. Some people complained of a metallic taste in their mouths and suffered from diarrhoea. By the time the disease ran its course, over 40 people died and over 100 were severely disabled. The people affected by the disease lived in a relatively small area, and their diet mostly came from fish harvested from Minamata Bay.
The disease was eventually traced to a vinyl chloride factory on Minamata Bay that used mercury in its production processes. Inorganic mercury was released as waste into the bay, and it was believed that the mercury would not get into the food chain. However, the inorganic mercury was converted by bacterial activity in Minamata Bay to methyl mercury. Methyl mercury readily passes through cell membranes and is transported throughout the body by red blood cells. It can enter and damage brain cells. The harmful effects of methyl mercury depend on a number of factors that include the amount of exposure and intake, the duration of the exposure, and the species affected. The effects of the mercury are often delayed from several weeks to months in people from the time of ingestion. Furthermore, if the intake of mercury ceases, some of the symptoms may disappear, but others are difficult to reverse.
The disease of the dancing cats eventually became known as Minamata disease, and nearly 800 people were officially recognized as having the disease but as many as several thousand may have been involved. The mercury pollution in the bay ceased in 1968. As recently as the 1990s, some of the people afflicted by the disease were still being compensated for damages.
Arsenic is an example of a highly toxic natural metal that is found in soil, rock, and water. There are many industrial and commercial uses of arsenic compounds, including the processing of glass, pesticides, and wood preservatives. Arsenic may enter our water supplies through a number of processes, including natural rain, snow melt, or groundwater flow. It may also be released with industrial waste water and agricultural processes. Finally, it may be released through the production of pesticides, the burning of fossil fuels, and as a by-product of mining.
Arsenic has been known as a deadly poison since ancient times, and, more recently, it has been recognized that elevated levels of arsenic in drinking water may cause a variety of health problems that affect organs such as the bladder, lung, and kidney. It may also cause disease to the central nervous system. Finally, arsenic is known to be a carcinogen (capable of causing or promoting cancer).
The occurrence of arsenic in drinking water is now recognized as a global problem. It certainly is not found in all water supplies, but it is found in many around the world. For example, arsenic in groundwater in Bangladesh has affected many millions of some of the poorest people on Earth. Ongoing research has the objective of identifying those locations where arsenic pollution occurs and of developing appropriate technology or methods to avoid or reduce the hazard of exposure to arsenic.
Radioactive Waste
Radioactive waste in water may be a dangerous pollutant. Environmentalists are concerned about the possible effects of long-term exposure to low doses of radioactivity to people, other animals, and plants.
Sediment
Sediment consists of unconsolidated rock and mineral fragments, the smallest of which range in size from sand particles to very small silt- and clay-sized particles. It is these small particles that cause most sediment pollution problems. Sediment is our greatest water pollutant by volume; it is clearly a resource out of place. It depletes soil, a land resource; can reduce the quality of the water resource it enters; and may deposit undesired materials on productive crop lands or on other useful land.
Thermal Pollution
Thermal pollution is the artificial heating of waters, primarily by hot-water emission from industrial operations and power plants. Heated water causes several problems. First, heated water contains less oxygen than cooler water; even water only several degrees warmer than the surrounding water holds less oxygen. Second, warmer water favours different species than does cooler water and may increase the growth rates of undesirable organisms, including certain water plants and fish. In some cases, however, the warm water may attract and allow better survival of certain desirable fish species, particularly during the winter.


