Igneous Rocks are formed by crystallization from a liquid, or magma. They include two types
Magmas are less dense than surrounding rocks, and will therefore move upward. If magma makes it to the surface it will erupt and later crystallize to form an extrusive or volcanic rock. If it crystallizes before it reaches the surface it will form an igneous rock at depth called aplutonic or intrusive igneous rock. |
Types of Magma
Chemical composition of magma is controlled by the abundance of elements in the Earth. Si, Al, Fe, Ca, Mg, K, Na, H, and O make up 99.9%. Since oxygen is so abundant, chemical analyses are usually given in terms of oxides. SiO2 is the most abundant oxide.
|
Summary Table
| ||||||
| Magma Type | Solidified Volcanic Rock | Solidified Plutonic Rock | Chemical Composition | Temperature | Viscosity | Gas Content |
| Mafic or Basaltic | Basalt | Gabbro | 45-55 SiO2 %, high in Fe, Mg, Ca, low in K, Na | 1000 - 1200 oC | Low | Low |
| Intermediate or Andesitic | Andesite | Diorite | 55-65 SiO2 %, intermediate in Fe, Mg, Ca, Na, K | 800 - 1000 oC | Intermediate | Intermediate |
| Felsic or Rhyolitic | Rhyolite | Granite | 65-75 SiO2 %, low in Fe, Mg, Ca, high in K, Na | 650 - 800 oC | High | High |
Origin of Magma
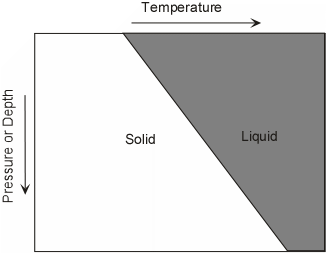
As we have seen the only part of the earth that is liquid is the outer core. But the core is not likely to be the source of magmas because it does not have the right chemical composition. The outer core is mostly Iron, but magmas are silicate liquids. Thus magmas DO NOT COME FROM THE MOLTEN OUTER CORE OF THE EARTH. Thus, since the rest of the earth is solid, in order for magmas to form, some part of the earth must get hot enough to melt the rocks present. We know that temperature increases with depth in the earth along thegeothermal gradient. The earth is hot inside due to heat left over from the original accretion process, due to heat released by sinking of materials to form the core, and due to heat released by the decay of radioactive elements in the earth. Under normal conditions, the geothermal gradient is not high enough to melt rocks, and thus with the exception of the outer core, most of the Earth is solid. Thus, magmas form only under special circumstances. To understand this we must first look at how rocks and mineral melt.As pressure increases in the Earth, the melting temperature changes as well. For pure minerals, there are two general cases. |
|  |
| 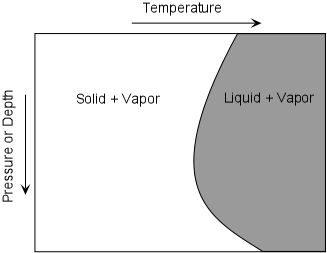 |
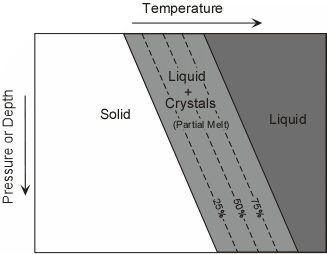
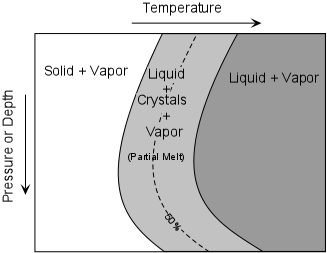
Since rocks mixtures of minerals, they behave somewhat differently. Unlike minerals, rocks do not melt at a single temperature, but instead melt over a range of temperatures. Thus, it is possible to have partial melts from which the liquid portion might be extracted to form magma. The two general cases are: |
|  |
|  |
| Three ways to Generate MagmasFrom the above we can conclude that in order to generate a magma in the solid part of the earth either the geothermal gradient must be raised in some way or the melting temperature of the rocks must be lowered in some way. The geothermal gradient can be raised by upwelling of hot material from below either by uprise solid material (decompression melting) or by intrusion of magma (heat transfer). Lowering the melting temperature can be achieved by adding water or Carbon Dioxide (flux melting). |
| Decompression Melting - Under normal conditions the temperature in the Earth, shown by the geothermal gradient, is lower than the beginning of melting of the mantle. Thus in order for the mantle to melt there has to be a mechanism to raise the geothermal gradient. Once such mechanism is convection, wherein hot mantle material rises to lower pressure or depth, carrying its heat with it. | 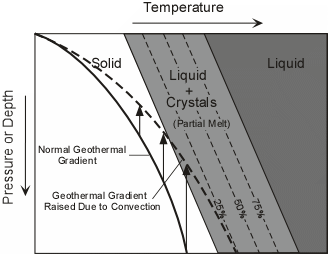 |
| If the raised geothermal gradient becomes higher than the initial melting temperature at any pressure, then a partial melt will form. Liquid from this partial melt can be separated from the remaining crystals because, in general, liquids have a lower density than solids. Basaltic magmas appear to originate in this way. Upwelling mantle appears to occur beneath oceanic ridges, at hot spots, and beneath continental rift valleys. Thus, generation of magma in these three environments is likely caused by decompression melting. | |
| Transfer of Heat- When magmas that were generated by some other mechanism intrude into cold crust, they bring with them heat. Upon solidification they lose this heat and transfer it to the surrounding crust. Repeated intrusions can transfer enough heat to increase the local geothermal gradient and cause melting of the surrounding rock to generate new magmas. |
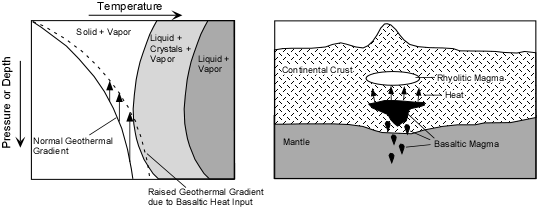 Transfer of heat by this mechanism may be responsible for generating some magmas in continental rift valleys, hot spots, and subduction related environments. Transfer of heat by this mechanism may be responsible for generating some magmas in continental rift valleys, hot spots, and subduction related environments.
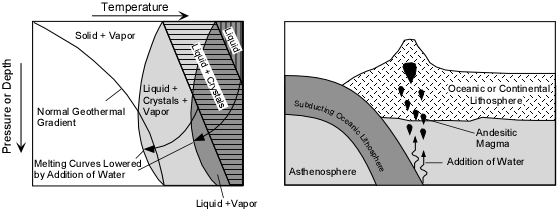
Flux Melting - As we saw above, if water or carbon dioxide are added to rock, the melting temperature is lowered. If the addition of water or carbon dioxide takes place deep in the earth where the temperature is already high, the lowering of melting temperature could cause the rock to partially melt to generate magma. One place where water could be introduced is at subduction zones. Here, water present in the pore spaces of the subducting sea floor or water present in minerals like hornblende, biotite, or clay minerals would be released by the rising temperature and then move in to the overlying mantle. Introduction of this water in the mantle would then lower the melting temperature of the mantle to generate partial melts, which could then separate from the solid mantle and rise toward the surface.
|
 |
Chemical Variability of Magmas
The chemical composition of magma can vary depending on the rock that initially melts (the source rock), and process that occur during partial melting and transport.Initial Composition of Magma The initial composition of the magma is dictated by the composition of the source rock and the degree of partial melting. In general, melting of a mantle source (garnet peridotite) results in mafic/basaltic magmas. Melting of crustal sources yields more siliceous magmas. In general more siliceous magmas form by low degrees of partial melting. As the degree of partial melting increases, less siliceous compositions can be generated. So, melting a mafic source thus yields a felsic or intermediate magma. Melting of ultramafic (peridotite source) yields a basaltic magma. Magmatic Differentiation But, processes that operate during transportation toward the surface or during storage in the crust can alter the chemical composition of the magma. These processes are referred to asmagmatic differentiation and include assimilation, mixing, and fractional crystallization. Assimilation - As magma passes through cooler rock on its way to the surface it may partially melt the surrounding rock and incorporate this melt into the magma. Because small amounts of partial melting result in siliceous liquid compositions, addition of this melt to the magma will make it more siliceous. 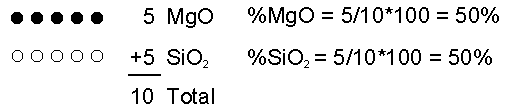 Now let's imagine I remove 1 MgO molecule by putting it into a crystal and removing the crystal from the magma. Now what are the percentages of each molecule in the liquid? 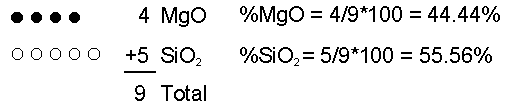 If we continue the process one more time by removing one more MgO molecule 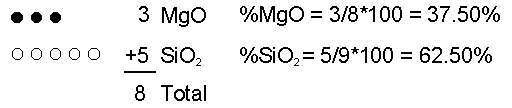 Thus, composition of liquid can be changed. |
Bowen's Reaction Series Bowen found by experiment that the order in which minerals crystallize from a basaltic magma depends on temperature. As a basaltic magma is cooled Olivine and Ca-rich plagioclase crystallize first. Upon further cooling, Olivine reacts with the liquid to produce pyroxene and Ca-rich plagioclase react with the liquid to produce less Ca-rich plagioclase. But, if the olivine and Ca-rich plagioclase are removed from the liquid by crystal fractionation, then the remaining liquid will be more SiO2 rich. If the process continues, an original basaltic magma can change to first an andesite magma then a rhyolite magma with falling temperature |
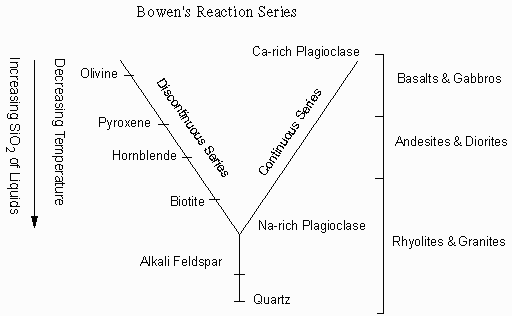 |
Igneous Environments and Igneous Rocks
The environment in which magma completely solidifies to form a rock determines:
The intrusive or plutonic environment is below the surface of the earth. This environment is characterized by higher temperatures which result in slow cooling of the magma. Intrusive or plutonic igneous rocks form here. Where magma erupts on the surface of the earth, temperatures are lower and cooling of the magma takes place much more rapidly. This is the extrusive or volcanic environment and results in extrusive or volcanic igneous rocks. Extrusive Environments When magmas reach the surface of the Earth they erupt from a vent called a volcano. They may erupt explosively or non-explosively.
|
|
| Tephra that falls from the eruption column produces a tephra fall deposit. | 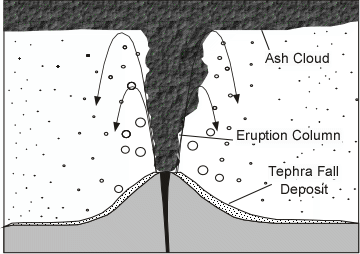 |
| If eruption column collapses a pyroclastic flow may occur, wherein gas and tephra rush down the flanks of the volcano at high speed. This is the most dangerous type of volcanic eruption. The deposits that are produced are called ignimbrites. | 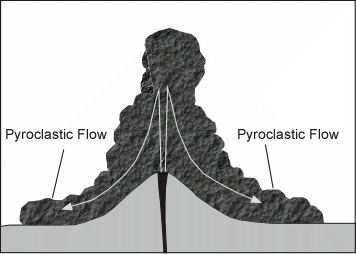 |
Intrusive Environments
Magma that cools at depth form bodies of rocks called intrusive bodies or plutonic bodies called plutons, from Greek god of the underworld - Pluto. When magma intrudes it usually affects the surrounding rock and is also affected by the surrounding rock. It may metamorphose the surrounding rocks or cause hydrothermal alteration. The magma itself may also cool rapidly near the contact with the surrounding rock and thus show a chilled margin next to the contact. |
| It may also incorporate pieces of the surrounding rocks without melting them. These incorporated pieces are called xenoliths (foreign rocks). Magma intrudes by injection into fractures in the rock and expanding the fractures. The may also move by a process called stoping, wherein bocks are loosened by magma at the top of the magma body with these blocks then sinking through the magma to accumulate on the floor of the magma body. | 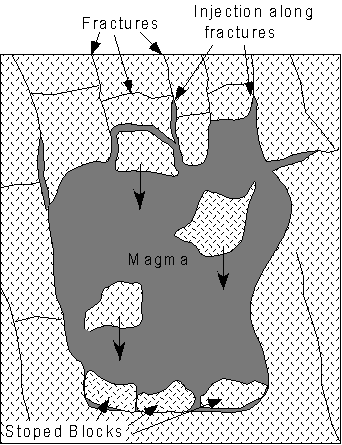 |
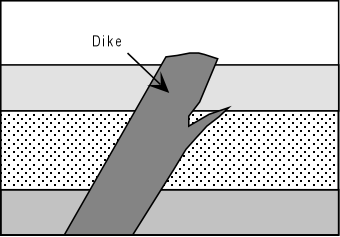
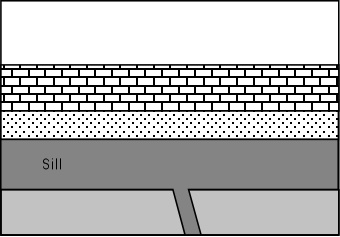
| In relatively shallow environments intrusions are usually tabular bodies like dikes and sills or domed roof bodies called laccoliths. | |
|  |
|  |
| 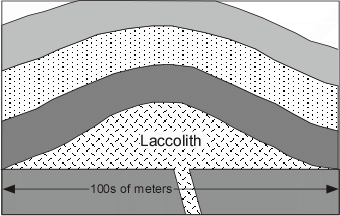 |
Deeper in the earth intrusion of magma can form bulbous bodies called plutons and the coalescence of many plutons can form much larger bodies called batholiths.
|
| 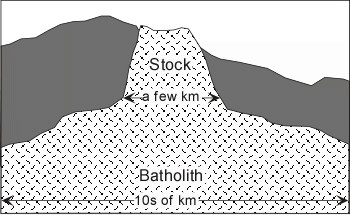 |
During a magmatic event there is usually a close relationship between intrusive activity and extrusive activity, but one cannot directly observe the intrusive activity. Only after erosion of the extrusive rocks and other rock above the intrusions has exposed the intrusions do they become visible at the earth's surface (see figure 6.10a in your text). |
| The rate of cooling of magma depends largely on the environment in which the magma cools. Rapid cooling takes place on the Earth's surface where there is a large temperature contrast between the atmosphere/ground surface and the magma. Cooling time for material erupted into air and water can be as short as several seconds. For lava flows cooling times are on the order of days to weeks. Shallow intrusions cool in months to years and large deep intrusions may take millions of years to cool. | |
Because cooling of the magma takes place at a different rate, the crystals that form and their interrelationship (texture) exhibit different properties.
|  |
| 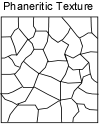 |
| 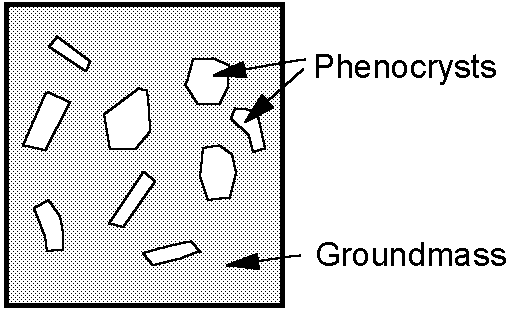 |
Classification of Igneous Rocks
Igneous rocks are classified on the basis of texture and chemical composition, usually as reflected in the minerals that from due to crystallization. You will explore the classification of igneous rocks in the laboratory portion of this course.
|
Extrusive/Volcanic Rocks
Basalts, Andesites, and Rhyolites are all types of volcanic rock distinguished on the basis of their mineral assemblage and chemical compostion (see figure 6.13 in your text). These rocks tend to be fine grained to glassy or porphyritic. Depending on conditions present during eruption and cooling, any of these rock types may form one of the following types of volcanic rocks.
|
|
| Intrusive/Plutonic Igneous Rocks Shallow intrusions like dikes and sills are usually fine grained and sometimes porphritic because cooling rates are similar to those of extrusive rocks. Classification is similar to the classification for volcanic/extrusive rocks. Coarse grained rocks, formed at deeper levels in the earth include gabbros, diorites, and granites. Note that these are chemically equivalent to basalts, andesites, and rhyolites, but may have different minerals or different proportions of mineral because their crystallization history is not interrupted as it might be for extrusive rocks (see figure 6.13 in your text). Pegmatites are very coarse grained igneous rocks consisting mostly of quartz and feldspar as well as some more exotic minerals like tourmaline, lepidolite, muscovite. These usually form dikes related to granitic plutons.
Distribution of Igneous Activity
Igneous activity is currently taking place as it has in the past in various tectonic settings. These include diverging and converging plate boundaries, hot spots, and rift valleys.
Divergent Plate Boundaries
At oceanic ridges, igneous activity involves eruption of basaltic lava flows that form pillow lavas at the oceanic ridges and intrusion of dikes and plutons beneath the ridges. The lava flows and dikes are basaltic and the plutons mainly gabbros. These processes form the bulk of the oceanic crust as a result of sea floor spreading. Magmas are generated by decompression melting as hot solid asthenosphere rises and partially melts.
Convergent Plate Boundaries
Subduction at convergent plate boundaries introduces water into the mantle above the subduction and causes flux melting of the mantle to produce basaltic magmas. These rise toward the surface differentiating by assimilation and crystal fractionation to produce andesitic and rhyolitic magmas. The magmas that reach the surface build island arcs and continental margin volcanic arcs built of basalt, andesite, and rhyolite lava flows and pyroclastic material. The magmas that intrude beneath these arcs can cause crustal melting and form plutons and batholiths of diorite and granite
Hot Spots
As discussed previously, hot spots are places are places where hot mantle ascends toward the surface as plumes of hot rock. Decompression melting in these rising plumes results in the production of magmas which erupt to form a volcano on the surface or sea floor, eventually building a volcanic island. As the overriding plate moves over the hot spot, the volcano moves off of the hot spot and a new volcano forms over the hot spot. This produces a hot spot track consisting of lines of extinct volcanoes leading to the active volcano at the hot spot. A hot spot located beneath a continent can result in heat transfer melting of the continental crust to produce large rhyolitic volcanic centers and plutonic granitic plutons below. A good example of a continental hot spot is at Yellowstone in the western U.S. Occasionally a hot spot is coincident with an oceanic ridge. In such a case, the hot spot produces larger volumes of magma than normally occur at ridge and thus build a volcanic island on the ridge. Such is the case for Iceland which sits atop the Mid-Atlantic Ridge.
Rift Valleys
Rising mantle beneath a continent can result in extensional fractures in the continental crust to form a rift valley. As the mantle rises it undergoes partial melting by decompression, resulting in the production of basaltic magmas which may erupt as flood basalts on the surface. Melts that get trapped in the crust can release heat resulting in melting of the crust to form rhyolitic magmas that can also erupt at the surface in the rift valley. An excellent example of a continental rift valley is the East African Rift.
Large Igneous Provinces
In the past, large volumes of mostly basaltic magma have erupted on the sea floor to form large volcanic plateaus, such as the Ontong Java Plateau in the eastern Pacific. Such large volume eruptions can have affects on the oceans because they change the shape of ocean floor and cause a rise in sea level, that sometimes floods the continents. The plateaus form obstructions which can drastically change ocean currents. These changes in the ocean along with massive amounts of gas released by the magmas can alter climate and have drastic effects on life on the planet.
|





























